
已知Wnt信號傳導參與幹細胞 (stem cell) 的早期發育,細胞維持與再生,以及癌細胞 (cancer) 的生成,Wnt信號傳導在這些生長和維持的過程中起重要的作用。
特別的是Wnt3a是維持腸上皮細胞中Lgr5+幹細胞增殖的必需生態成分 (essential niche component),並且用於生產各種消化器官,例如小腸,大腸,胃,胰臟和肝。
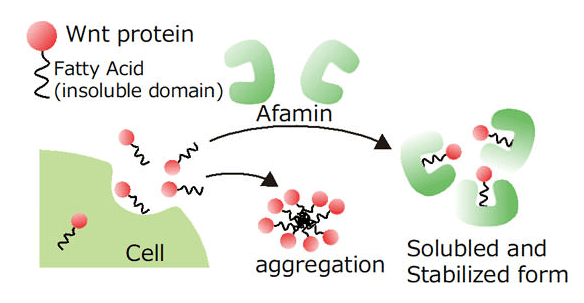
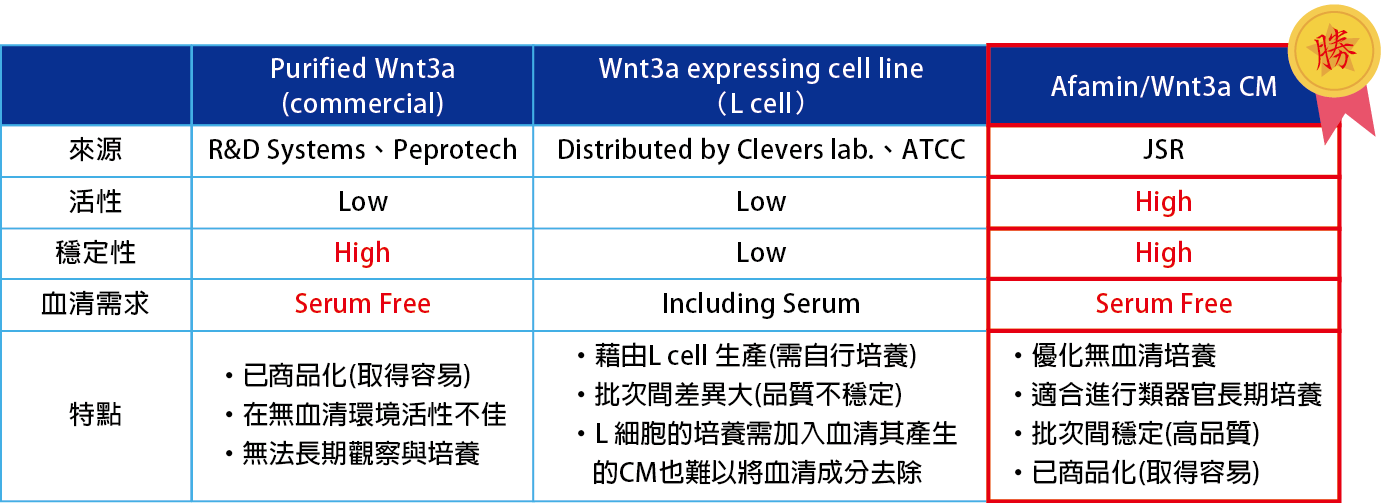
但因Wnt3a是一種脂溶性蛋白質,在無血清培養基 (serum-free medium) 中會形成團聚而不能充分發揮其活性。
使用Afamin和Wnt3a複合物進行培養,讓您無須加入FBS,又可改善Wnt3a活性/穩定度不佳的問題,適用長期培養,相信能為您的實驗帶來更好的結果。
- 產品特色
- 產品比較
- 產品規格
- 參考文獻
-
高穩定性 Stabilized Wnt3a
-
高活性 High Activity
-
無血清培養 Serum Free
使用Afamin/Wnt3a CM協助類器官長期培養
以大腸類器官為例,與使用市售Wnt3a或含有血清的Wnt3a的培養方式相比,Afamin / Wnt3a CM可以進行更加長期穩定地培養。
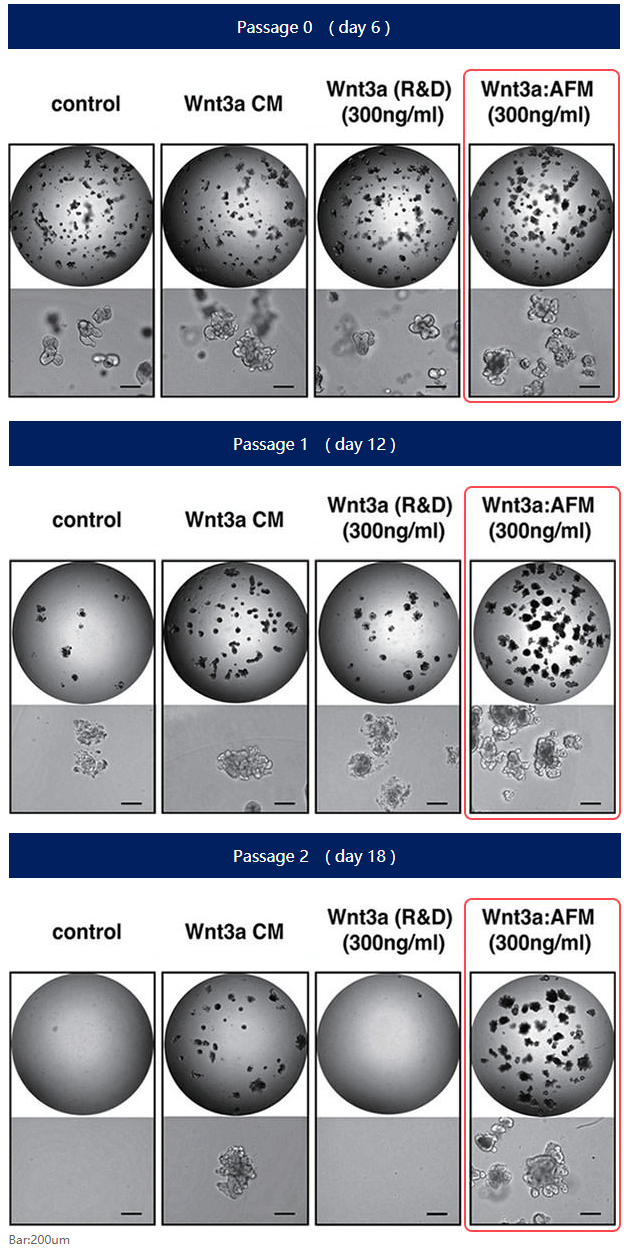
Afamin有助於提高Wnt3a的穩定性
Afamin作為血清的替代物可以穩定Wnt3a的活性,在無血清培養基中也能維持較高的穩定性,有助於類器官的長期培養。
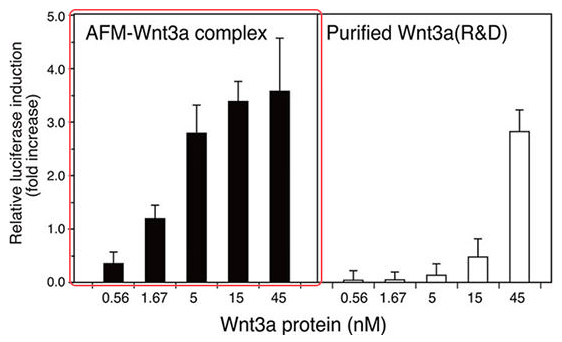
Afamin / Wnt3a CM具有較高的活性
通過TOP FLASH實驗發現,與市售純化的Wnt3a蛋白相比,Wnt3a在與Afamin 結合後具有更高的活性。
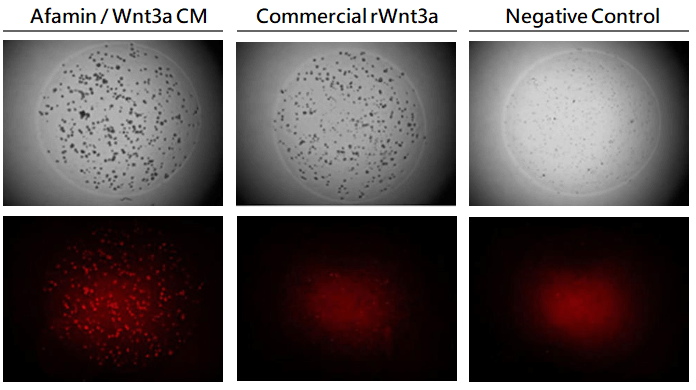
使用Afamin/Wnt3a CM持續培養LGR5+細胞
在培養Lgr5啟動子調節下的td Tomato螢光蛋白的類器官株時,同時使用Afamin/Wnt3a CM和市售的Wnt3a培養基進行培養比較,結果使用Afamin/Wnt3a CM培養的LGR5+細胞存活率更高。
以往在類器官培養使用的Wnt3a,一般是市售的重組Wnt3a或是由ATCC等處以可表達Wnt3a的細胞進行培養後得到含有Wnt3a的Conditioned Medium。多數情況下,使用這些Wnt3a進行類器官培養時,由於Wnt的活性並不好,容易導致培養失敗。
此外,在使用細胞株表達蛋白的Conditioned Medium時,為了維持Wnt3a活性,大多會在培養基中加入血清,但是血清的存在,會對類器官的增殖及培養產生一定影響。 Afamin/Wnt3a CM已被驗證在無添加血清時也能保持Wnt的活性,優化類器官的培養。
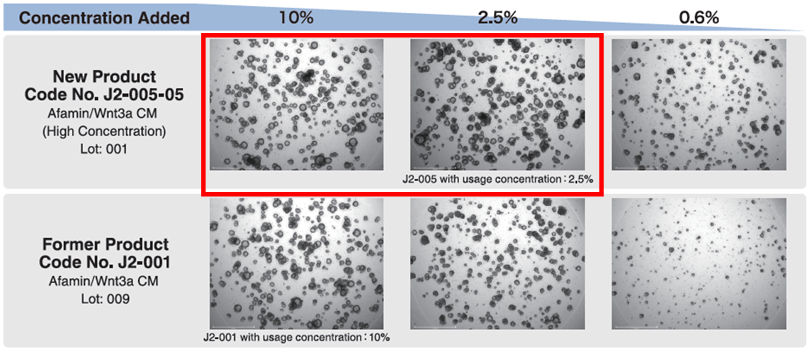
J2-005-05為更高濃度與活性的新一代產品
新產品(J2-005-05)和原產品(J2-001)用於培養人類小腸類器官。
原產品(J2-001)在濃度分別為 10% 與 2.5% 時,原產品(J2-001)濃度 2.5% 時類器官生長有減少趨勢。
使用新產品(J2-005-05),即使添加濃度為 2.5%,仍能維持的類器官生長。
|
Product Code |
Product Name |
Main Components |
Size |
Solvent |
|
J2-001 |
Afamin/Wnt3a CM |
Mouse Wnt3a Human Afamin |
10mL |
Advanced D-MEM/F-12 |
|
J2-002 |
Recombinant Afamin/Wnt3a |
Mouse Wnt3a Human Afamin |
60ug/300uL |
20mM Tris-HCI (pH7.4), 150 mM NaCI |
|
J2-005-05 |
Afamin/Wnt3a CM (High Concentration) |
Mouse Wnt3a Human Afamin |
5mL |
Expi293TM Expression Medium |
|
Organ |
Title |
PMID |
||
|
Intestine |
Normal tissue |
E. Mihara, et al., Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin., eLife 5 (2016) |
||
|
S. Sugimoto, et al., Reconstruction of the human colon epithelium in vivo., Cell Stem Cell 22 (2018) |
||||
|
S. Sugimoto, et al., Organoid Derivation and Orthotopic Xenotransplantation for Studying Human Intestinal Stem Cell Dynamics., Methods Mol Biol 2171 (2020) |
||||
|
N. Sasaki, et al., Development of a Scalable Coculture System for Gut Anaerobes and Human Colon Epithelium., Gastroenterology 159 (2020) |
||||
|
Zwiggelaar RT et al. LSD1 represses a neonatal/reparative gene program in adult intestinal epithelium. Sci Adv. 2020 Sep 11;6(37):eabc0367. doi: 10.1126/sciadv.abc0367. Print 2020 Sep |
||||
|
M. Fujii, et al., Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition., Cell Stem Cell 23 (2018) |
||||
|
S. Sugimoto, et al., An organoid-based organ-repurposing approach to treat short bowel syndrome., Nature 592 (2021) |
||||
|
Ulcerative colitis |
K. Nanki, et al., Somatic inflammatory gene mutations in human ulcerative colitis epithelium., Nature 577 (2020) |
|||
|
Tumor |
H. Oshima, et al., Stat3 is indispensable for damage-induced crypt regeneration but not for Wnt-driven intestinal tumorigenesis., FASEB J 33 (2019) |
|||
|
K. Kawasaki, et al., Chromosome Engineering of Human Colon-Derived Organoids to Develop a Model of Traditional Serrated Adenoma., Gastroenterology 158 (2020) |
||||
|
T. De Oliveira, et al., Effects of the Novel PFKFB3 Inhibitor KAN0438757 on Colorectal Cancer Cells and Its Systemic Toxicity Evaluation In Vivo., Cancers (Basel) 13 (2021) |
||||
|
T. Nishina, et al., Interleukin-11-expressing fibroblasts have a unique gene signature correlated with poor prognosis of colorectal cancer., Nat Commun 12 (2021) |
||||
|
Stomach |
Normal tissue |
K. Nanki, et al., Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis., Cell 174 (2018) |
||
|
Tumor |
K. Nanki, et al., Divergent routes toward Wnt and R-spondin niche independency during human gastric carcinogenesis., Cell 174 (2018) |
|||
|
K. Togasaki, et al., Wnt Signaling Shapes the Histologic Variation in Diffuse Gastric Cancer., Gastroenterology 160 (2021) |
||||
|
Pancreas |
Normal |
T. Seino, et al., Human pancreatic tumor organoids reveal loss of stem cell niche factor dependence during disease progression., Cell Stem Cell 22 (2018) |
||
|
Tumor |
K. Miyabayashi, et al., Intraductal transplantation models of human pancreatic ductal adenocarcinoma reveal progressive transition of molecular subtypes., Cancer Discov 10 (2020) |
|||
|
JS. Roe, et al., Enhancer Reprogramming Promotes Pancreatic Cancer Metastasis., Cell 170 (2017) |
||||
|
H. Tiriac, et al., Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment., Gastrointest Endosc 87 (2018) |
||||
|
H. Tiriac, et al., Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer., Cancer Discov 8 (2018) |
||||
|
Ureteric Bud |
iPSC- Derived |
S. Mae, et al., Expansion of Human iPSC-Derived Ureteric Bud Organoids with Repeated Branching Potential., Cell Reports 32 (2020) |
||
|
Ovary |
Tumor |
Y. Nanki, et al., Patient-derived ovarian cancer organoids capture the genomic profiles of primary tumours applicable for drug sensitivity and resistance testing., Scientific Reports 28 (2020) |
||
|
Gastroenteropancreatic neuroendocrine neoplasms(GEP-NENs) |
K. Kawasaki, et al., An Organoid Biobank of Neuroendocrine Neoplasms Enables Genotype-Phenotype Mapping., Cell 183 (2020) |
|||
|
Alveolus |
Normal tissue |
T. Ebisudani, et al., Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening., Cell Rep 35 (2021) |
||
|
Salivary gland |
Normal tissue |
D. Kim, et al., 3D Organoid Culture From Adult Salivary Gland Tissues as an ex vivo Modeling of Salivary Gland Morphogenesis., Front Cell Dev Biol 9 (2021) |
||
