
- (QualTex)
- (GenCure)
- (IPOC)
 |
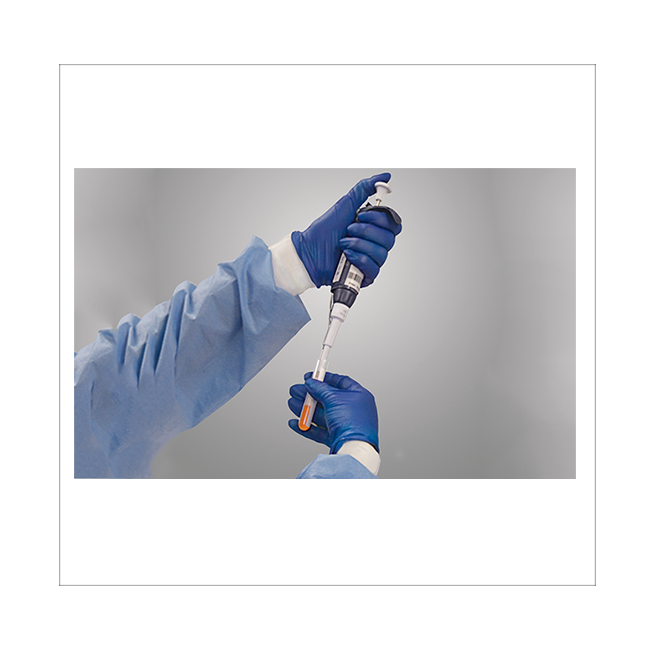
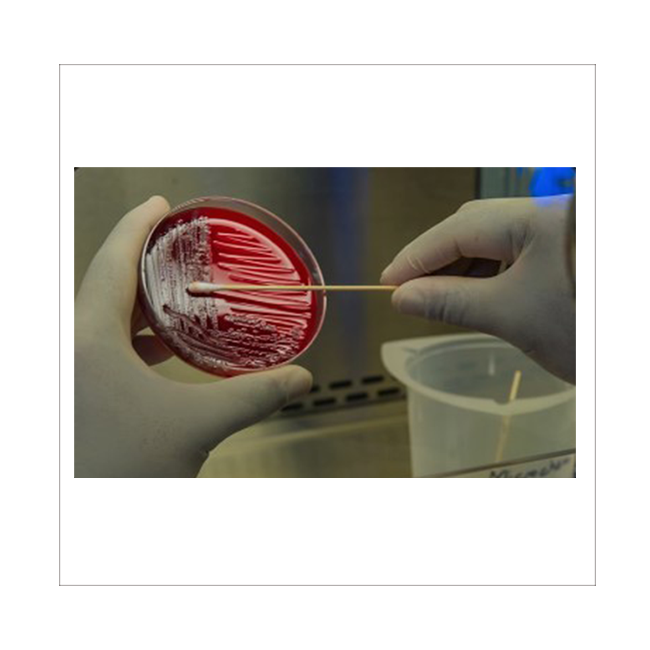
The main service items are "Specific Pathogen Screening (Donor Screening)" and "Product Testing (Product Testing)". QualTex uses FDA approved reagents (FDA Approval Kit) or internal certification (Internal Validation Assay) to conduct a number of tests, in addition to In addition to providing GMP-level test results (CoA), we can also help you answer questions raised by the regulatory agency (FDA).
 |
 |
 |
||
| Donor Screening | Product Testing | Microbiology |
 |
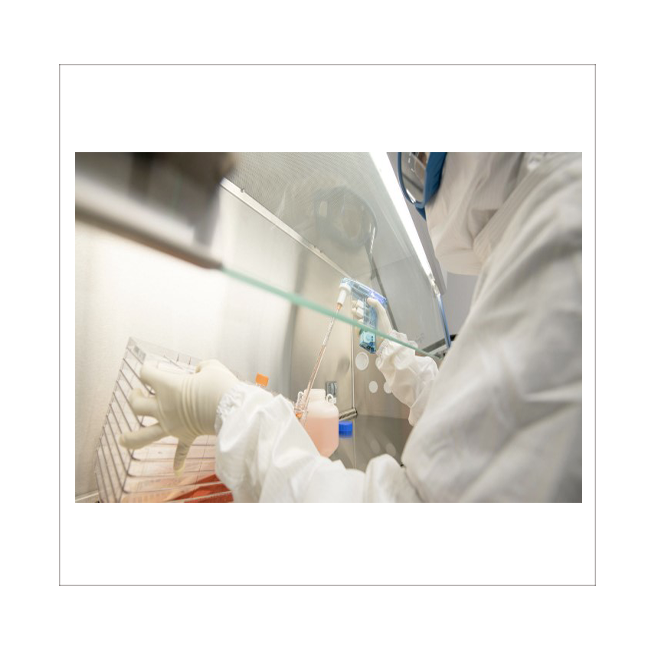
Entrusted by the U.S. government, we are committed to developing mass production platforms for various cell types (MSC, iPS, Immune Cell), with cGMP-level production sites, with the ultimate goal of clinical development and product commercialization.
 |
||||
| CDMO Service |
 |
International Point of Care Incorporated is headquartered in Toronto, Canada, with a 36,000-square-foot factory that complies with GMP and ISO13485 standards. They focus on the field of point-of-care testing, providing Primecare blood separation membranes, antibodies, recombinant proteins, customized freeze-drying services, etc., and are committed to providing more complete and diversified solutions to global customers.
 |
 |
 |
||
| 抗體 / 抗原 | 血液分離膜 | 凍乾服務 |
